FDA Approves Civilian Use of New Medical Device to Treat Gunshot Wounds
OutdoorHub Reporters 12.09.15
Military technology always spills over into the civilian market. We can thank the Department of Defense for countless innovations from the internet to the GPS you are probably use everyday.
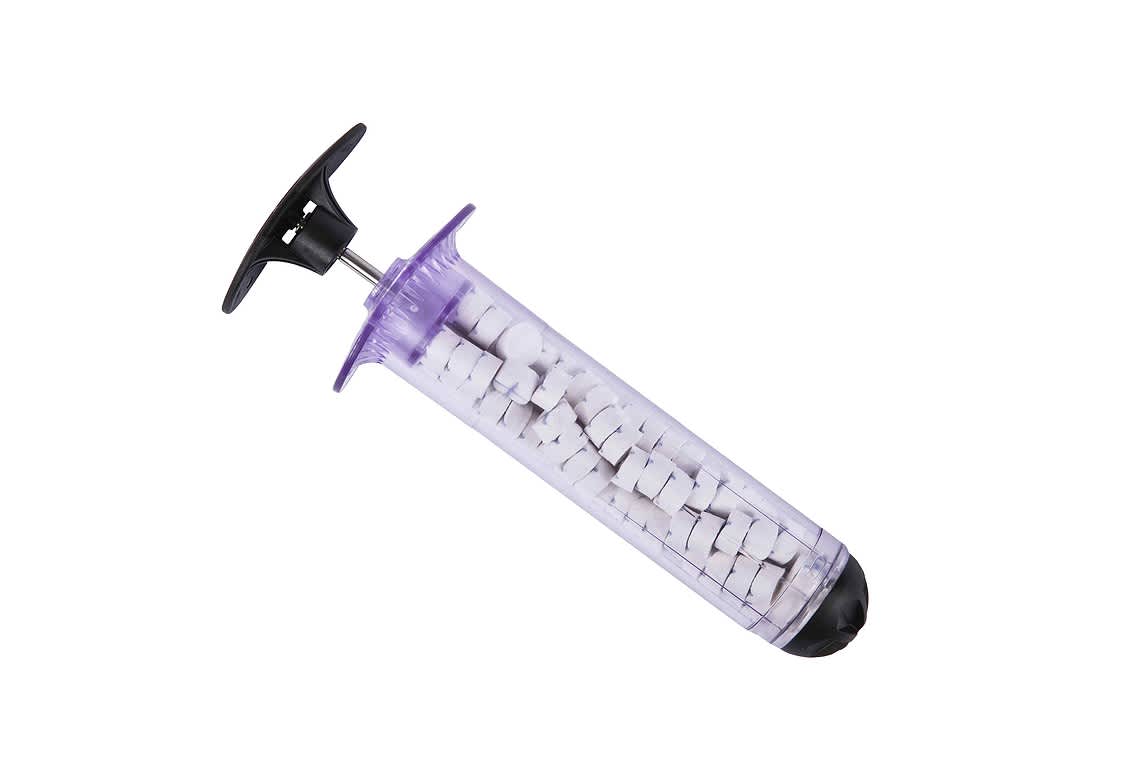
Now a medical device, named the XSTAT 30 and designed to treat soldiers with gunshot wounds, has been approved for civilian use by the Food and Drug Administration (FDA).
The approval comes after being used by the military since 2013. The device works by injecting a number of small sponges into a wound. The sponges quickly absorb blood and then expand, providing hemostatic pressure on the wound to prevent further bleeding. The device is especially useful for wounds in areas that cannot easily be treated with a tourniquet.
According to the United States Army Institute of Surgical Research, 30 to 40 percent of civilian deaths by traumatic injury are the result of hemorrhaging, and 33 to 56 percent occur before the patient reaches a hospital.
This device could help prevent many of those deaths and it has applications for sportsmen as well. The XSTAT 30 would make a range first aid kit much better equipped and it would also be a welcome addition to a hunter’s pack.